Dr. Paul Perry Named a Center of Excellence Physician for Carpal Tunnel Release Using Ultrasound Guidance by The Institute of Advanced Ultrasound Guided Procedures
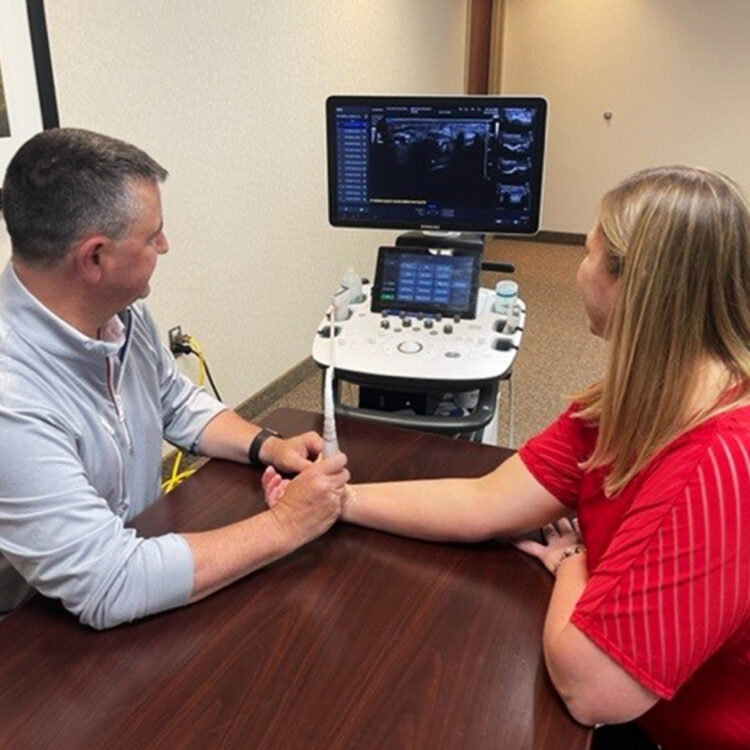
The Institute of Advanced Ultrasound Guided Procedures has named Dr. Paul Perry of Tri-State Orthopaedic Surgeons in Evansville, Indiana a national Center of Excellence Physician for carpal tunnel release (CTR) with UltraGuideCTR™ and real-time ultrasound guidance.
Dr. Perry has performed more than 500 carpal tunnel release procedures with ultrasound guidance, helping patients resume normal activities in much shorter time than with traditional CTR approaches.
The minimally invasive technique, which involves one small wrist incision, typically uses a bandage rather than sutures to close the incision and over-the-counter pain medication, such as Ibuprophen, is usually all that is needed to manage post-operative pain. Most patients can return to work and normal activities within 3–6 days.
“This technique fits perfectly within our practice, which focuses entirely on issues that arise from a patient’s shoulder to their fingertips”
Dr. Paul Perry, Tri-State Orthopaedic Surgeon
“This technique fits perfectly within our practice, which focuses entirely on issues that arise from a patient’s shoulder to their fingertips,” says Dr. Perry. “We know how much our patients depend on the use of their hands and I’m pleased to be able to offer this minimally invasive approach to relieving their carpal tunnel pain.”
Dr. Perry says his patients are often thrilled to learn that the procedure does not require general anesthesia and they can usually resume normal activities within days versus weeks or months.
“We are pleased to honor Dr. Perry in this way because of his commitment to offering innovative, minimally invasive techniques that can really make a difference in patients’ lives,” says Dr. Jay Smith, Chief Medical Officer at Sonex Health and The Institute of Advanced Ultrasound Guided Procedures.

An estimated 13 million adults in the United States1 suffer from carpal tunnel syndrome (CTS), a nerve disorder that causes numbness, tingling, and pain in the hands and fingers. Left untreated, CTS can cause long-term damage and debilitation.
It has been estimated that more than 2.7 million CTS patients are indicated for carpal tunnel release surgery2, yet only 580,000 procedures are performed each year3. The most common reasons for declining CTR surgery are fear of the surgery and concerns about recovery time.4,5 Providing a solution that can address these concerns will help to close the treatment gap and improve patients’ quality of life.
ABOUT ORTHOPAEDIC HAND SURGEON DR. PAUL PERRY
Dr. Paul E. Perry is an orthopaedic hand surgeon who practices in Evansville, Indiana. His practice focuses exclusively on problems of the upper extremity including the shoulder, elbow, wrist, and hand. His training and many years of clinical experience include introducing a number of innovative surgical techniques to the Tri-State region, including carpal tunnel release with ultrasound guidance and others. He received his medical degree from Wright State University Boonshoft School of Medicine, and served his residency at University of Florida Health.
###
ABOUT SONEX HEALTH
Founded in 2014, Sonex Health’s mission is to be the world leader in ultrasound guided surgery by delivering physicians innovative therapies that reduce invasiveness, improve safety, and reduce the cost of care. With a strong focus on entrapment neuropathy, Sonex Health’s first proprietary technology — developed by Dr. Darryl E. Barnes and Dr. Jay Smith at the world-renowned Mayo Clinic — is UltraGuideCTR (formerly referred to as SX-One Micro-Knife), which may be utilized with or without ultrasound guidance to perform carpal tunnel release. Sonex Health’s second proprietary technology is UltraGuideTFR, for the treatment of trigger finger, also known as stenosis tenosynovitis.
ABOUT THE INSTITUTE OF ADVANCED ULTRASOUND GUIDED PROCEDURES
Founded in 2018 to support the Sonex Health mission and clinical excellence, The Institute of Advanced Ultrasound Guided Procedures is focused on innovation supported by robust clinical research, and world-class professional education and training that transforms the treatment experience for patients, providers and payers.
References:
1. Papanicolaou GD, et al. The prevalence and characteristics of nerve compression syndromes in the general population. J Hand Surg 2001;26A:460-6.
2. Atroshi I, et al. Severe carpal tunnel syndrome potentially needing surgical treatment in a general population. J Hand Surg 2003;28A:649-44.
3. Fajardo M, et al. Incidence of carpal tunnel release: trends and implications with the United States ambulatory care setting. J Hand Surg 2012;37A:1599-1605.
4. Gong HS, Baek GH, Oh JH, Lee YH, Jeon SH, Chung MS. Factors affecting willingness to undergo carpal tunnel release. JBJS. 2009;91(9):2130-2136.
5. Sonex Health Market Research “Why not Undergo CTR?”
