Closing the treatment gap
Traditional open and endoscopic carpal tunnel release (CTR) procedures are effective but may result in a lengthy recovery period and a large and sometimes painful scar.4-7
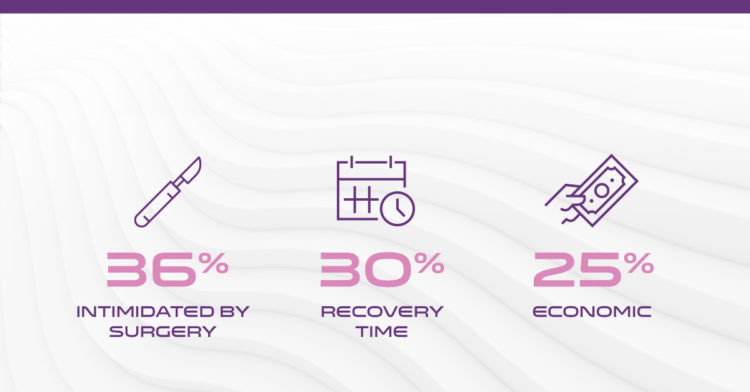
Understanding why patients elect to avoid traditional CTR surgery will help close the treatment gap.8,9
*Image Reference: Reasons for Declining CTR Procedures: 36% Intimidated by Surgery, 30% Recovery Time and 25% Economic8,9
Reasons for declining CTR procedures